Why Does Silicon Dioxide Have High Melting Point
Glass melts at a lower temperature than silicon dioxide. Melting and boiling points.
![]()
Macromolecules Covalent Network Solids Last Part Of Topic Ppt Download
The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds.
. It also has the highest melting point because the covalent bonds between them is the strongest. Other substances are added to silicon dioxide to make glass. Covalent bonds stronger in SiO2 than in SiCl4 so more energy isneeded to break bonds.
Covalent bonds are much stronger than Van der Waals forces and so require much more energy to overcome and this leads to the. Sulfur Trioxide has a simple molecular structure meaning it has Van der Waals forces between molecules. The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds ionic or covalent operating in three dimensions.
Why does silicon dioxide have such a high melting point. SiO2 has higher melting point. The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds ionic or covalent operating in three dimensions.
SiO2 has a high melting point. A lot of energy is needed to break the strong covalent bonds throughout the structure. 1 The melting point of the elements going across the Periodic Table from sodium to argon A increases steadily.
Why does SiO2 have such a high melting point. More force is required to break down. In respect to this why does silicon have a higher melting point than sodium.
Because the silicon and oxygen atoms are held by strong intermolecular forces. Diamond has a much shorter C-C bond length 154 pm and stronger bonds 348 kJmol. Melting and boiling points.
A lot of energy is needed to separate the atoms. Thus the melting temperature of silicon is higher as it exists as a giant covalent structure so strong covalent bonds have to be broken in order to melt it whereas phosphorus exists as a simple covalent structure so weaker London forces have to broken in order to melt it. This causes diamond and graphite to have high melting points.
The melting of SiO2 requires breaking of a large number of covalent bonds and hence its melting point is high. 7 280 Fahrenheit The ultimate melting point of diamond is about 4 027 Celsius 7 280 FahrenheitNov 4 2015. Why diamond has higher melting point than Aluminium.
And a bond strength of 318 kJmol. Diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms forming. Melting and boiling points of these oxides are much lower than those of the metal oxides or silicon dioxide.
This is the reason why diamond has a high melting point. Muxakara and 11 more users found this answer helpful. It has a very high melting point as the ionic bond is very strong between them.
Glass is made from silicon dioxide. Silicon carbide has a C-Si bond length of 186 pm. A lot of energy is needed to break the strong covalent bonds throughout the structure.
The carbon atoms are held together by strong covalent bonds which. Silicon Dioxide has a macromoleculargiant covalent structure which means it has covalent bonds between all atoms in its structure. Why does silicon dioxide have a high melting point.
SiO2 has a high melting point 1700 degrees Celsius. Melting and boiling points. Does diamond have a high melting point.
Both diamond and graphite have a giant molecular structure. Why does silicon dioxide have such a high melting point. Why does silicon dioxide have a high melting point.
This is because covalent bonds are strong. Why melting point of diamond is higher than silicon carbide. Why does silicon dioxide have a high melting point.
Why does silicon dioxide have such a high melting point. The intermolecular forces binding one molecule to its neighbors are van der Waals dispersion forces or dipole-dipole. Why Argon has low melting point.
Each carbon is covalently bonded to three others so there is one electron delocalised per carbon atom which is free to move between the layers and carry charge. The molecular oxides Phosphorus sulfur and chlorine form molecular oxides. What is diamond melting point.
Silicon has a very high melting point due to its giant covalent structure. This is due to the strength of Si-O-Si binds in the lattice. The giant structures the metal oxides and silicon dioxide will have high melting and boiling points because a lot of energy is needed to break the strong bonds ionic or covalent operating in three dimensions.
A Silicon dioxide has a very high melting point. Similarly why does silicon have a higher melting point than phosphorus. Silicon has a very high melting point due to its giant covalent structure.
This is due to the strength of Si-O-Si binds in the lattice. Silicon dioxide is a giant covalent structure. The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds ionic.
Since Diamonds contain more covalent bond than Silicon Dioxide and these bonds requires heatenergy to break Diamond has a higher melting point than Silicon dioxide. Why Does Carbon Have A High Melting PointCarbon atoms have four unpaired electrons and can form four covalent bonds. Why do diamond and graphite both have high melting points.
The oxides of phosphorus sulphur and chlorine consist of individual molecules - some small and simple. A lot of energy is needed to break the strong covalent bonds throughout the structure. Silicon has a very high melting point due to its giant covalent structure.
Also why does phosphorus oxide have a low melting point. Also The Bond length between Carbon-Carbon atoms is lesser than that of Silicon making it harder to break the bonds. Melting and boiling points.
Since covalent bonds require more energy to overcome than van der waals SiO2 requires a higher temperature to melt. Why does silicon dioxide have a high melting point.

Why Is Carbon Dioxide A Gas While Silicon Dioxide A Solid Quora
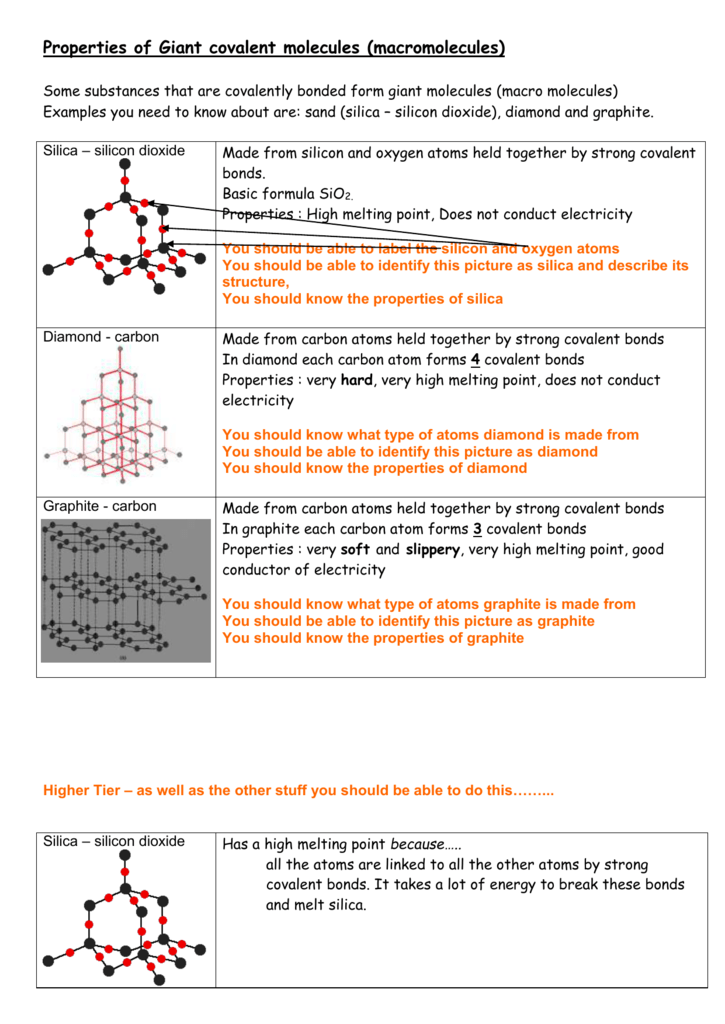
Properties Of Giant Covalent Molecules Macromolecules

Based On Their Structures Explain Why Sodium Oxide Silicon Dioxide And Carbon Dioxide Have Different Melting Points Here Are 6 Real Student Answers Ppt Download
0 Response to "Why Does Silicon Dioxide Have High Melting Point"
Post a Comment